Thermodynamics Review
Temperature:
The temperature of two objects “a” and “b” in thermal contact will reach thermal equilibrium eventually. This is the “0th” law of thermodynamics.
Equation of state: T,P,V,N
what is N? Avogadro number?
- N
is the number of particles (usually atoms or molecules) of the gas.
Ideal Gas:
Van Der Wahls Gas:
Work
is the work done on a system
is this below equation equiv to the above?

Where is an infinitesimal change in distance, and thus where A is the area of the cylinder above.
infinitesimal work can be given as a derivative of volume.
We can use the equation of state:
Quasi-static Process: . I think
For an Ideal gas compressed at constant temperature:
If we go from some volume to some volume
If , then it is not a state function
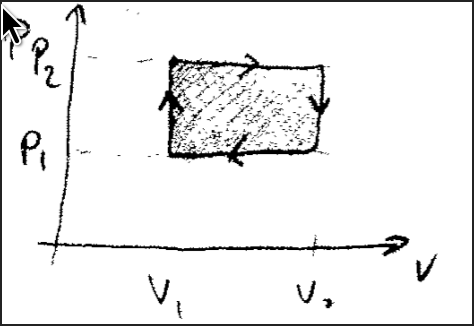
Is this the total work done? I believe so.
The first law of thermodynamics
Given a thermally isolated system: (adiabatic process)
E (energy) is a state function, uniquely determined by T,P,N…
In the case of thermal contact, where is not 0, this is heating/cooling.
Q is temprature? or heat? so is heating/cooling?
(not a state function)
Equation of state for the energy
If N is constant (not adding or removing mass from the system )
Ideal Gas: (mono-atomic gas)
Van Der Whals gas:
Notice how energy does not depend on volume in the ideal gas case.
Using statistical mechanics to derive this from “microcopies”
what is a microcopy
Isothermal Process for ideal gas, where T is constant
isothermal means constant temperature?
This is the work done from a change in volume, i.e compression or expansion.
Heat Capacity and Enthalpy
Heat capacity (aka specific heat) is the amount of heating needed to change the temperature by a unit amount.
For a constant volume:
and
As an example: The monoatomic ideal gas:
Enthalpy
Enthalpy is thermodynamic potential? Denoted by H, where H is
and
You can use enthalpy to find heat capacity? For constant pressure:
General Relationship between Constant pressure and volume
This is constant pressure and temperature enthalpy.
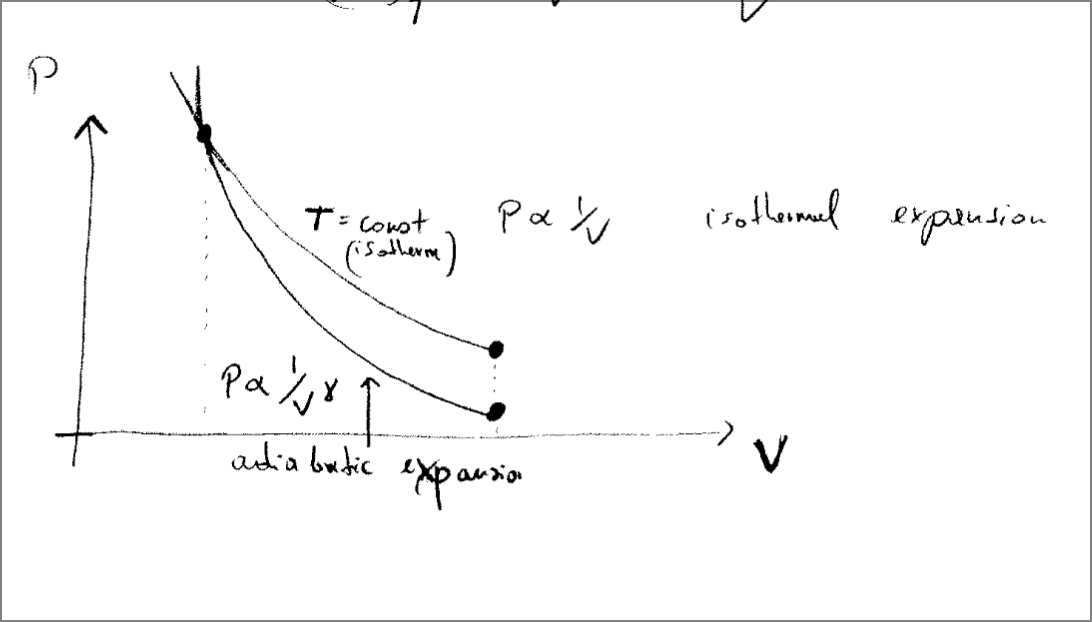
Adiabatic Processes
An adiabatic process is a process that excludes heat transfer or exchange. Perfectly insulated.
The definition of an adiabatic process:
There is no energy exchange due to temperature difference.
This is a “quasistatic” process. Quasistatic adiabatic process.