- Dr Monika Szumilo
- Worked on graphene!!
Main devices to be covered: Diodes Photovoltaics Transistors Thermoelectrics
Organic Semiconductors:
What are organic semiconductors?
- Carbon based
- Typically polymers, such as plastics, rubbers, etc
- Aka Plastic Electronics
Helps overcome problems with traditional semiconductors, such as silicon. Silicon is high-quality and easy to obtain, but:
- very energy-intensive and expensive to refine
- Doesn’t work well for large area applications.
- Very heavy.
OSC’s are cheap to make, flexible, biocompatible, and can be made in large sizes, but:
- slower then silicon
- sensitive to water, UV, o2
OSC’s fall into two classes, polymer and molecular.
How do OSC’s work?
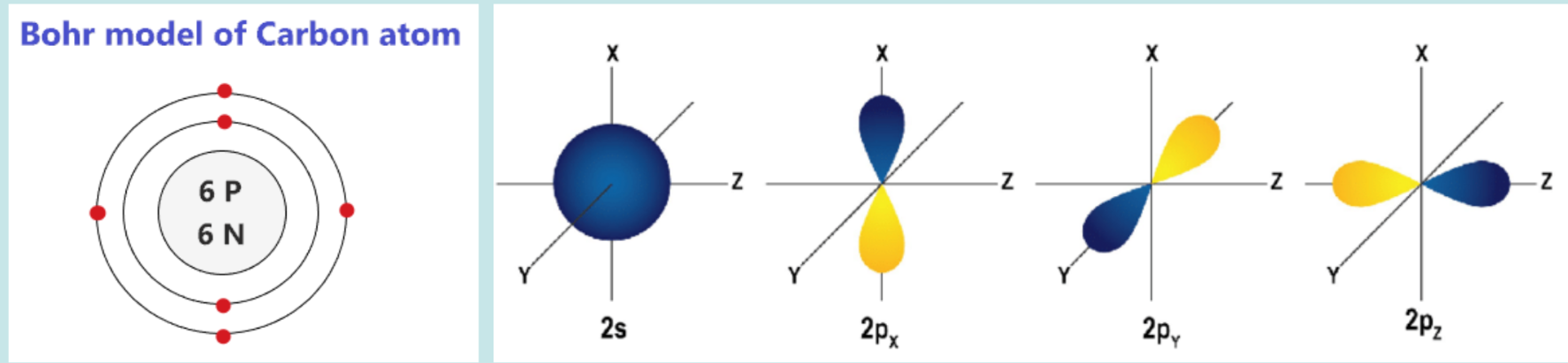
Carbon has 6 electrons, stored in 3 orbitals.
These electrons can be stored in a “hybridized” orbital, between the S and P orbitals.
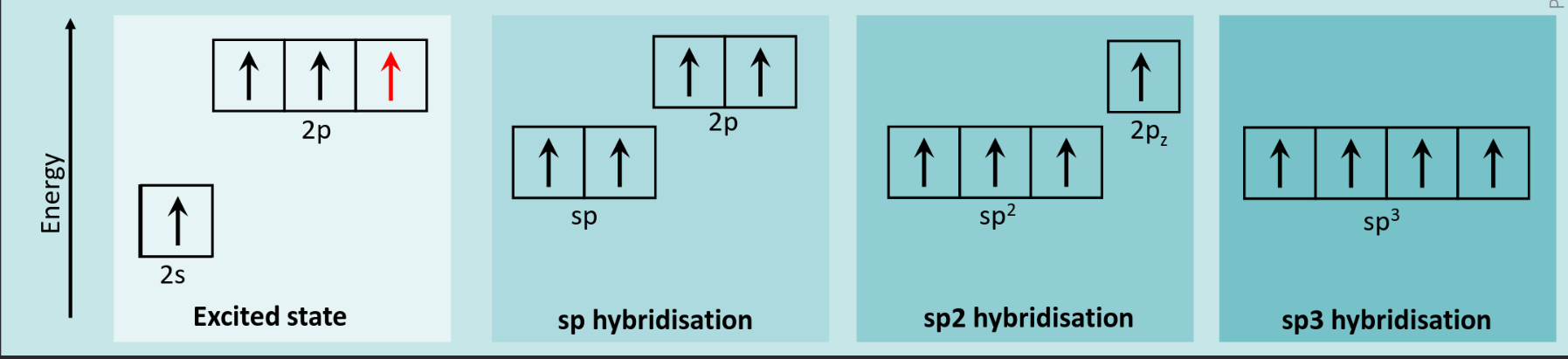
We are most interested in the sp2 hybridization, because with this the remaining electron in the p_z orbital has interesting properties.
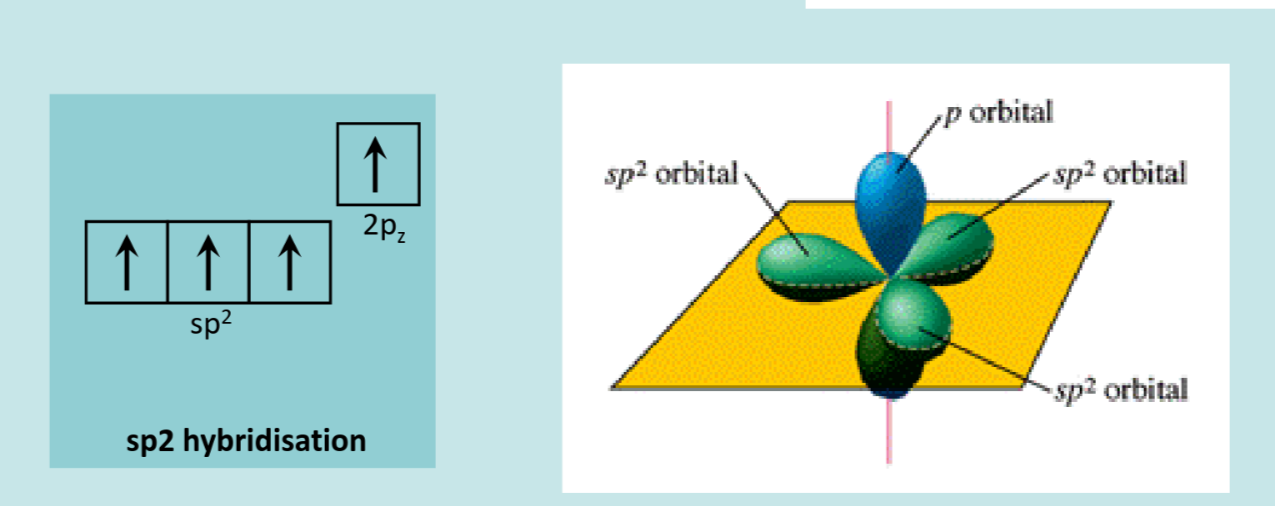
Carbon Atom bonding:
Sigma bonds:
- Strong
- Single direction
- can rotate
- Responsible for carbon chaining
bonds:
- Weaker
- Prevents rotation
- Most importantly: Allows de-localisation of orbitals, which allows carbon to conduct electricity.
Adjustable band gaps:
- The presence of pi bonds allows for adjustable band gaps. HOMO: Highest Occupied Molecular Orbital LUMO: Lowest unoccupied molecular orbital
In summary: Size of bandgap in molecule depends on two major things:
- Molecule composition
- This is length of
- Planarity
Key question:
Q: How does the concept of hybridization contribute to the understanding of organic semiconductors?
- It explains the formation of delocalized electron clouds.
- It predicts the geometry of the molecules.
- It helps to understand the energy levels of molecular orbitals.
Polarons:
Peierl’s transition, aka dimerization:
- When carbon atoms come in pairs?
Key Question:
Q: What is the main driving force for Peierl’s transition in conjugated polymers? A: To achieve a lower energy state.
Q: How does the presence of polarons affect the optical properties of an organic semiconductor? A: It leads to the formation of new absorption bands.
Excitons:
- Quasi-particle
- Comes in Wannier and Frenkel flavours
Wannier Excitons:
- Weak
- 10 milli- eV
- Large distance
- Found in inorg. semiconductors Frenkel Excitons:
- Strong
- Binding energy 0.2-1 eV
- Small distance
- Typically in OSC’s
- Easily recombines
Singlet-triplet splitting:
- Ground state is typically a singlet
- The exchange energy is the difference between first excited singlet state and first excited triplet state
- Triplet states are useless, and cant be converted to singlet states.
- Active area of research due to being source of inefficiency
- What does “manifold” mean in this context
Spectroscopy
An exciton is a bound state of an electron and a hole